Introduction
The hematopoietic system is especially vulnerable to chemotherapeutic treatment-related long-term consequences, including clonal and malignant hematopoiesis. These diseases are caused by the acquisition of driver mutations in hematopoietic stem and progenitor cells (HSPCs). An increased rate of mutation accumulation, as identified in HSPCs of patients who receive chemotherapeutic treatment, can therefore result in disrupted blood production. Accordingly, childhood cancer survivors have a five-fold increased risk of secondary cancers, amongst which therapy-related myeloid neoplasms (t-MN) are one of the most prevalent. Here, we aim to increase the understanding of the origin of t-MN through the characterization of the short- and long-term mutational consequences of chemotherapy in different hematopoietic stem and progenitor cell types.
Methods
We studied the genome-wide mutation burden in the HSPC compartment at the time of an initial cancer diagnosis, following chemotherapeutic treatment, and upon a second cancer diagnosis. We performed whole-genome sequencing (WGS) of single HSPCs, including hematopoietic stem cells (HSCs, CD34+CD38-CD45RA-CD90+), multipotent progenitor cells (MPPs, CD34+CD38-CD45RA-CD90-), common myeloid progenitor cells (CMPs, CD34+CD38+CD45RA-), and granulocyte-monocyte progenitor cells (GMPs, CD34+CD38+CD45RA+). In order to obtain enough DNA for WGS, we performed clonal expansion of single cells or primary template-directed amplification of the DNA of one cell. Next, we normalized the mutation burden in each cell to the expected number of mutations (Osorio et al. Cell Reports 2018). In addition, we performed both whole genome and transcriptome sequencing of individual HSCs/MPPs.
Results
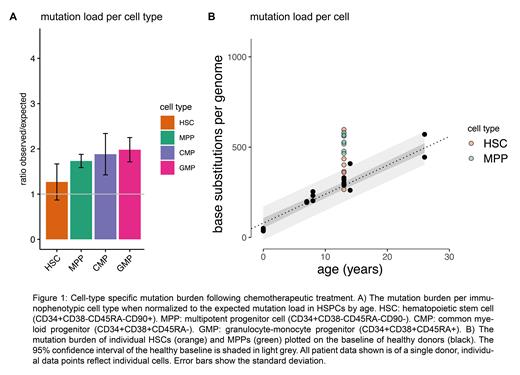
First, we investigated pediatric ten t-MN patients with a latency between the initial and second cancer diagnosis spanning 1-12 years. We applied WGS to clonally expanded HSPCs (CD34+CD38-CD45RA-) and found that the relative increase in mutation burden in HSPCs is restricted to the first five years after initial treatment. To gain more insight into the heterogeneous HSPC compartment, we subsequently investigated cell-type specific mutational loads based on immunophenotypic markers. So far, we have included three pediatric cancer patients with comparable treatment regimens and diagnoses, namely medium risk acute lymphoblastic leukemia. Here, we found that the mutation burden is lower in HSCs when compared to the progenitor cell types (Figure 1A). When comparing the mutation load in the HSCs to the linear mutation accumulation observed in HSCs and MPPs of healthy donors, we identified a fraction of cells overlapping with the expected mutation load based on the age of the patient (Figure 1B). These data suggest that HSCs are more protected against chemotherapy-induced mutagenesis than progenitor cells, although this protective effect varies between individual cells. Therefore, we also performed both whole genome and transcriptome sequencing from a single cell, to characterize the transcriptional state of HSCs/MPPs showing a relatively high or low mutation load within their respective cell type.
Conclusions
Our results indicate that primitive hematopoietic stem cells are more protected against chemotherapy-induced mutations than the more proliferative progenitor cells. In addition, we find that in t-MN patients with a long latency (>5 years), the mutation load in HSPCs returns to the expected baseline. Based on our data, we hypothesize that the more damaged progenitor cells may be replaced over time, fueled by the undamaged primitive stem cell pool. Thus, our results may explain why the risk of t-MN drops 5-7 years after treatment end.
Disclosures
No relevant conflicts of interest to declare.